
The electronic configuration of the first 30 elements is shown in the table below. The electronic configuration of oxygen, for example, can be written as 1s 2 2s 2 2p 4 It is written in superscript with the shell number, the name of the subshell, and the total number of electrons present in the subshell. The number of electrons present in the subshell is represented by notation.
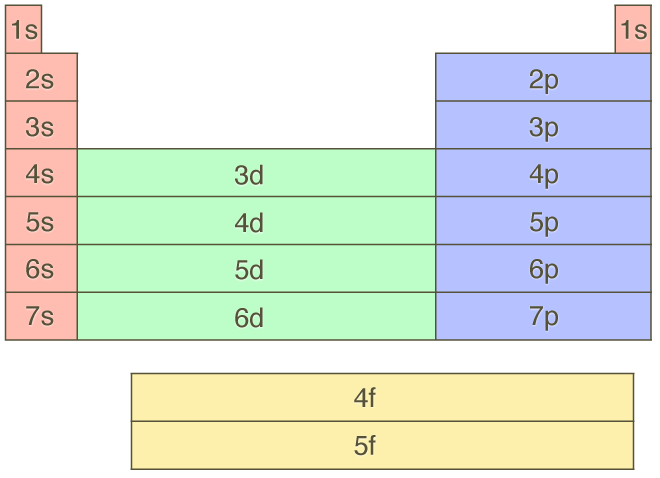
As a result, the singly filled electrons all have the same spin, ensuring the orbital stability. However, according to Hund’s rule, each orbital is partially filled before electron pairing. In the case of Aufbau’s principle, electrons are filled in orbitals from lowest to highest energy levels. Hund’s rule is an extension of Aufbau’s principle. This means that if one electron is spin-up, the other must be spin-down. Every orbital has the capacity to hold two electrons, and even those two electrons have different spins. Pauli’s Exclusion Principle:Īccording to Pauli’s exclusion principle, no two electrons in an atom can have the same four quantum numbers. Aufbau’s principle, Pauli’s exclusion principle, and Hund’s rule are used to write the electronic configuration.Īufbau’s Principle states that electrons are filled in shells in the following order of energy levels: 1s, 2s, 2p, 3s, 3p, 4s, 4p, 5s, 4d, 5p, 6s, 5d, 6p, 7s, 5f, 6d, 7p, and so on.

The orbitals are denoted by the letters s, p, d, and f, and the maximum number of electrons present in these orbitals is 2, 6, 10, and 14. The number of electrons present in each subshell surrounding the nucleus of an atom is represented by its electronic configuration.


 0 kommentar(er)
0 kommentar(er)
